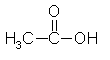
Acetic acid
| 원료 | acetaldehyde; oxygen | ||||||||||||
| 생산물 | acetic acid | ||||||||||||
| 적용 | To produce acetic acid by oxidation of acetaldehyde with oxygen. The process is characterized by: high yield, low utility consumption and low investment cost.<br> The catalyst is recycled without any treatment and any losses. From the crude acetic acid the final product of high purity is distilled in three columns under atmospheric pressure. Byproducts are not obtained. | ||||||||||||
| 설명 | Description: Acetaldehyde and oxygen serve as feedstock for the production of acetic acid. Acetaldehyde is oxidized (1) into acetic acid by oxygen in the presence of a catalyst containing manganese acetate. The reaction may be summarized as follows: The reaction takes place at normal pressure and at a temperature of approx. 60℃. The heat of reaction is removed by a cooling system. The crude acetic acid obtained after separation from the off-gases is distilled in three columns(2, 3, 4) under normal pressure. In the first column the crude acetic acid is separated from the low-boiling components. The residual acetic acid contained in the head product of the first column is drained off as bottom product in the second column and is recycled into the reactor. The light ends distilled overhead are led to the flare. The catalyst contained in the bottoms of the third column is returned to the reaction without further treatment and without losses. The final product acetic acid is distilled overhead.
Raw materials and utilities (per 1,000 kg acetic acid):
|
||||||||||||
| Properties in KDB |
Component Names and Formula
Click here to find more informations from KDB.
|