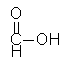
Formic acid
| 원료 | Methanol; CO | ||||||||||||
| 생산물 | formic acid | ||||||||||||
| 적용 | To produce high purity formic acid from impure CO gas and H2O. | ||||||||||||
| 설명 | Description: A dilute, impure or pure carbon monoxide gas is reacted (1) with methanol to produce methylformate. After separating (2) the CO stack gas, the methylformate is hydrolyzed (3) and the products are separated (4,5,6) to produce pure formic acid and methanol. The methanol is recycled to the first stage of this process.
HCOOCH3 + H2O <==> HCOOH + CH3OH Net reaction = CO + H2O <==> HCOOH
Yield on CO gas depends on the quality of the gas but yields are high. Utility consumptions: per 100 lb of product:
Product quality: 80 to 98% product acid. |
||||||||||||
| Properties in KDB |
Component Names and Formula
Click here to find more informations from KDB.
|